Abstract
Introduction: Daratumumab (D) is a human monoclonal antibody targeting CD38 that is approved as monotherapy and in combination with immunomodulatory drugs (lenalidomide or pomalidomide) or a proteasome inhibitor (bortezomib) for RRMM. The combination of D and bortezomib plus dexamethasone (DVd) was compared to Vd in CASTOR (ClinicalTrials.gov NCT02136134), a randomized, multicenter, phase 3 study in patients with RRMM. In the primary analysis, 29 deaths in DVd and 36 deaths in Vd were observed (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.47-1.26; Palumbo et al, N Engl J Med 2016;375(8):754-66). Here, we provide an updated analysis of overall survival (OS) in CASTOR.
Methods: Eligible patients received ≥1 prior line of therapy and were randomly assigned to receive 8 cycles (every 3 weeks) of Vd (V 1.3 mg/m2 subcutaneously on Days 1, 4, 8, and 11; d 20 mg orally or intravenously (IV) on Days 1-2, 4-5, 8-9, and 11-12) with or without D (16 mg/kg IV once weekly in Cycles 1-3, every 3 weeks for Cycles 4-8, and every 4 weeks thereafter until disease progression). Patients who were refractory to bortezomib were ineligible. Progression-free survival (PFS) was the primary endpoint. OS was a secondary endpoint. Minimal residual disease (MRD) via next generation sequencing was assessed upon suspected complete response and at 6 and 12 months after the first dose at 3 sensitivity thresholds (10-4, 10-5, and 10-6) using the clonoSEQTM assay (Version 1.3, Adaptive Biotechnologies, Seattle, WA). A pre-specified interim analysis of OS was planned after 160 OS events had accrued.
Results : 251 patients received DVd and 247 patients received Vd. The median (range) age was 64.0 (30-88) years. Patients received a median of 2 (1-10) prior therapies; 61% received prior autologous stem cell transplant, 66% received prior bortezomib (with 13% receiving >1 prior bortezomib-containing regimen), 42% received prior lenalidomide, 48% received prior proteasome inhibitor plus immunomodulatory drug, 28% were refractory to lenalidomide, and 32% were refractory to their last line of therapy. The median duration of treatment with single-agent daratumumab after receiving 8 cycles of Vd was 11.9 months.
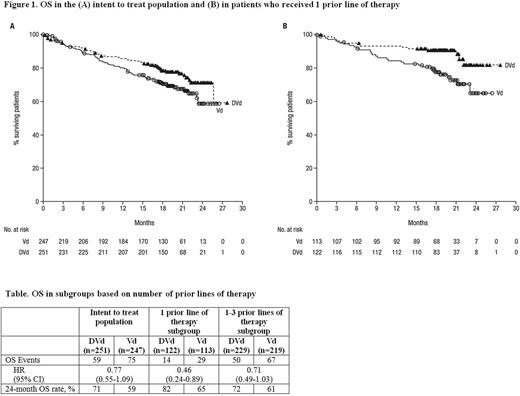
After median follow-up of 19.4 (range: 0-27.7) months, median OS has not been reached in either treatment group in the intent-to-treat (ITT) population (Figure 1A)andin patients who received 1 prior line of therapy (Figure1B). An exploratory analysis of the number of OS events and the 24-month OS rates revealed fewer deaths with DVd vs Vd in the ITT population and in subgroups based on the number of prior lines of therapy (Table). Among MRD-negative patients at the 10-5 sensitivity threshold, no OS events occurred in patients receiving DVd (n=29) versus 1 OS event being reported in the Vd arm (n=6). Among patients previously treated with bortezomib, the 24-month OS rate was 68% with DVd (n=162) vs 52% with Vd (n=164). Among patients refractory to lenalidomide, the 24-month OS rate was 62% and 47% with DVd (n=60) and Vd (n=81), respectively.
Conclusions: Updated OS data and additional subgroup analyses will be presented at the meeting based on a pre-specified analysis of OS after 160 OS events.
Lentzsch: British-Myers Squibb, Celgene, Janssen: Consultancy; Takeda: Speakers Bureau; Caelum BioSciences: Equity Ownership. Quach: Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria. Capra: Janssen: Speakers Bureau. Ovilla: Takeda Pharmaceutical Company: Consultancy, Honoraria. Thiyagarajah: Janssen: Employment. Amin: Janssen: Employment. Casneuf: Janssen: Employment. Sonneveld: Celgene Corporation, Amgen, Janssen, Karyopharm, PharmaMar, SkylineDx: Honoraria; Celgene Corporation, Amgen, Janssen, Karyopharm, SkylineDx, PharmaMar: Consultancy; Celgene, Amgen, Janssen, Karyopharm, Takeda: Consultancy, Honoraria, Research Funding. Schecter: Janssen: Employment. Hungria: Celgene, Roche, Takeda, Janssen, Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.